
"I am writing the story that will never end in my heart."

"I am writing the story that will never end in my heart."
|
|
Comments
Here are the 3 Comments (:1. Aleen Tan (27) I like the way she asnwered all the questions in the blog. The answers were detailed and well-explained. She stated clearly on what is what and besides that, she put in extra effort in finding the pictures that are relevant to the questions and she actually shared all her knowledge of this chapter in the blog.I like that for qns 5, besides saying Group 1, 2 and 3 are metals, she also added metals from other groups that is not from group 1,2 and 3 to make it more detailed. She never fails to put in examples and even though her answers were long, it was easy to understand. One mistake was that the charge outside should be + and not 1+. For her blog, it's very simple and i like it. She shared with us her achievements, favourites, hobby and hates and i'm sure she puts in alot of effort in it. In short, You did a great great great Job! :D 2. Wan Ling (16) I like that her answes were short, sweet and simple. Even though it was short, all the information was inside. I like that she includes pictures for almost all the questions to make her answer seem more interesting. One mistake was that is should be 1/1840 and not 1/1836. For her blog, her ownership for her blog is excellent as besides posting pictures and photos of her, she also posted videos that she likes from the internet! All the videos and photos she posted was interesting and i think we should learn from her. Overall, Well Done! ^.^ 3. Xue Li (7) I like that she had included pictures for almost all the questions but then, we should always put acknowledgement so please remember to put in order so that marks will not be deducted. You have also forgotten to put the drawings for sulfide ion to answer the questions. All points were included in the answers and i think nothing was left out, which is good. The blog is simple and it seems that she actually put in effort in doing the blog, for she also added in music. Anw, Good job!(:
Done!
 Okay, Chemistry Blog is done.! Done the blog is up to standard! 7 July is coming, which means its my DaJie's birthday (:
Fifth Question (:
5. Sodium is a metal and sulfur is a non-metal....why we classify them this way??Sodium is a metal as it has a valance electron of 1 that will be lost during a chemical reaction to make it stable and when it lost electron, it is positively charge. For sulfur it is a non-metal , it has a valance electron of 6 and it will gain 2 electrons during a chemical reaction to make it stable. As it gain electrons, it becomes negatively charge. In the Periodic Table, Sodium is being classified in Group (I) and they are metals. On the other hand, for Sulfur, it is being classified under Group (IV) which are non-metals. Therefore Sodium is able to conduct electricity as it is a metal and sulfur does not conduct electricity as it is a non-metal.
Fourth Question (:
4. Chlorine-35 atom and Chlorine-37 atom are called isotopes...Use these two examples to explain what is 'isotopes'. Isotopes are different atoms of the same element which have the same number of protons but different number of neutrons.
They have the same number of protons that is 17 but they do not have the same number of neutrons as chlorine 35 has 18 neutrons and chlorine 37 has 20 neutrons. As they both have the same number of protons but different number of neutrons, we can hence conclude that they are isotopes. Third Question (:
  In the above photo, it shows sulfur atom and sulfide ion. In sulfur atom, it has an electronic configuration of (2.8.6). This means that it is not stable as the valance shell only has 6 electrons and to make it stable, it must have 8 electrons. Therefore, in order to make it stable, it needs to gain 2 more electrons to make it 8 electrons to be stable. When it gain 2 electrons, the electronic configuration becomes (2.8.8) and it becomes a sulfide ion. As the overall is negatively charge, it is S2-. Second Question (:
2. Draw the atomic structure of a sodium atom and a sodium ion....explain why you draw it this way. Sodium ion can also be shown this way: 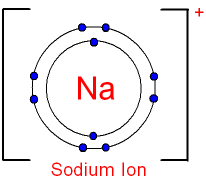 In Sodium atom, its electronic configuration is (2.8.1). Therefore, it will only have 1 electron in the valancy shell. To make it stable, we need to have 8 electron in one shell and hence, the 1 electron in the valancy shell is unstable. Since it only has 1 electron, it loses the electron to become stabilise and it becomes a sodium ion. As when an atom loses electron, it becomes an ion. When it loses electron, the overall ion will become positively charge and therefore, it becomes Na+ and it's in Group 1. First Question (:
1. What does an atom looks like? What are the sub-atomic particles inside it.....(talk about electrons, neutrons, protons, electron shells, nucleus....)Atoms are made up of three different particles. -Protons -Neutrons -Electrons These particles are even smaller than the atoms themselves. We call them sub- atomic particles. Protons and neutrons are tightly packed together in the center of an atom, forming the nucleus. Surrounding them are called the electrons.Below it shows the simplified structure of an atom.  Protons are positively charge (+) and electrons are negatively charge(-). Since they are both opposite charges, this enable them to cancel out each other and hence, resulting in electrical attraction between the protons and electrons. Unlike proton and electron, neutrons are neither positive or negative, they are neutral. Therefore : Proton
An atom also always has the same number of protons and electrons. If they do not have the same number, they are not call atom. But instead its an ion. For the circle around the nucleus, they are called the shells. The first shell can hold 2 electrons and from the second shell onwards, they are able to hold 8 electrons. Electrons also move rapidly around the nucleus. Chemistry Question
Questions that were given by Mr Tan : 2. Draw the atomic structure of a sodium atom and a sodium ion....explain why you draw it this way. 3. Draw the atomic structure of a sulfur atom and a sulfide ion....explain why you draw it this way. 4. Chlorine-35 atom and Chlorine-37 atom are called isotopes...Use these two examples to explain what is 'isotopes'. 5. Sodium is a metal and sulfur is a non-metal....why we classify them this way?? |
Info
Agnes, Fifteen, YHSS, 3E5 , YHCB; String Bass <3. Currently residing in Singapore, (: . I enjoy CHEMISTRY. :D Facebook Twitter Tagboard
Affiliates
Mr Tan
Ysabelle, 1
Cindy, 2
Grace, 4
Shin Yoong, 5
Ei Ei, 6
Xue Li, 7
Chelsea, 8
Janis, 9
Zi Qing, 10
Hwee Leng, 11
Shu Yu, 12
Jie En, 13
Siuk Geok, 15
Wan Ling, 16
Kai Qi, 18
Xue Ting , 19
Xin Hui , 21
Yi Hui , 22
Cek Min , 23
Su , 24
Ming Siew , 25
Yi Jin, 26
Aleen, 27
Wendy, 28
Yi Ting, 29
Michelle, 30
Clarence, 32
Xin Kang, 33
Fabian, 34
Helmi, 35
Jia Le, 36
Ze Wei, 37
Jun Wei, 38
Chun Boon, 39
Jun Jie, 40
Zane, 41
|